RIDITM: Rapid Infectious Disease Identification by Sequencing
Pan-Bacterial (Bacteria/Archaea) DNA Analysis AND Pan-Eukaryotic (Protozoa/Fungi) DNA Analysis
One test to detect all known bacteria, archaea, and, pathogenic fungi and protozoans.
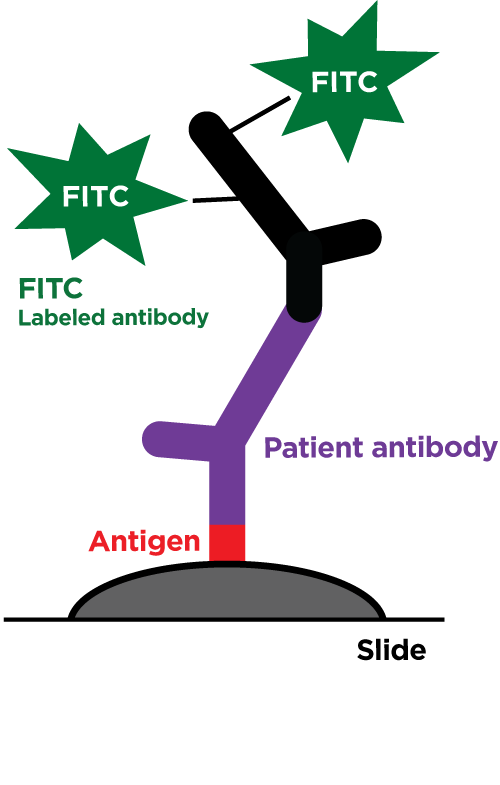
Our revolutionary RIDITM software screens many different sample types for the presence of all known bacteria, archaea, pathogenic fungi, and protozoans. Utilizing our proprietary Next Generation Sequencing chemistry and bioinformatics analysis, our RIDITM software is capable of identifying organisms at the species level or the nearest known relative. This test requires that the organisms be present in the sample provided and does not discriminate between viable or dead organisms. Potential novel organisms are flagged and the nearest known relative is identified. RIDITM is more sensitive and accurate than standard single/multi-organism PCR panels and fickle microbial culturing, reducing the risk of false positives, false negatives, and speculation over appropriate treatment. By identifying the specific organisms, RIDITM provides the critical data patients and providers need to make informed health decisions.
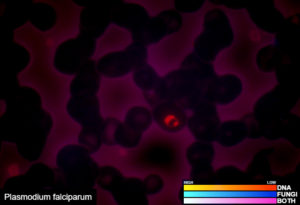
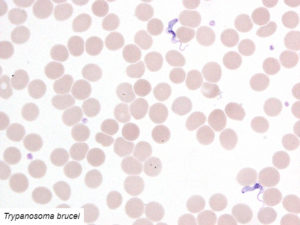
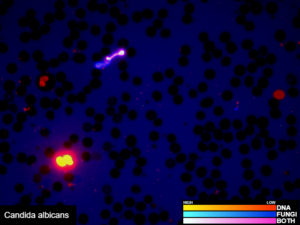
Examples of validated organisms include, but are not limited to: Staphylococcus, Streptococcus, Bartonella, Borrelia, Trypanosoma, Giardia, Acanthamoeba, Prototheca, Leishmania, Babesia, Cryptococcus, Cryptosporidium, Blastocystis, Entamoeba, etc. For more information about DNA sequencing, click here.