Introduction:
Lyme disease is a complex and devastating illness. Some of the most recent information regarding this disease is presented here. Test kits may be ordered using the “Order Test Kits” button at the top of the page or test kits may be requested by your healthcare provider.
Information
Lyme disease is a bacterial infection caused by the spirochete, Borrelia burgdorferi. Interestingly, other Borrelia species have been shown to cause similar diseases. These other species include: B. garinii, B. afzelii, and B. miyamotoi. Wilhelm Burgdorfer, a Swiss zoology and microbiology researcher, spent much of his later career at the Rocky Mountain Laboratory in Hamilton, Montana, and is credited with the discovery of Lyme disease. By using chemical staining and light microscopy techniques, William Burgdorfer was able to detect and study spirochetes that were found in deer ticks. Since that time at least 36 distinct species of Borrelia have been discovered, with at least 12 able to cause disease. Recent research has suggested that there are two main groups of Borrelia species with one group appearing to be responsible for Lyme or Lyme-like diseases and the other group responsible for relapsing fever related diseases [1].
The genomic sequence of B. burgdorferi strain B31 has a core genome of 910,725 base pairs with at least 17 additional genomic plasmids that add up to an additional 533,000 base pairs. Approximately 853 genes control cellular function and replication. At the time of B. burgdorferi sequencing in 1997, the majority of the 430 genes on the plasmids did not have any known function [2]. However, subsequent research has demonstrated that these plasmid-borne genes contribute to the disease causing nature of the organism [3,4].
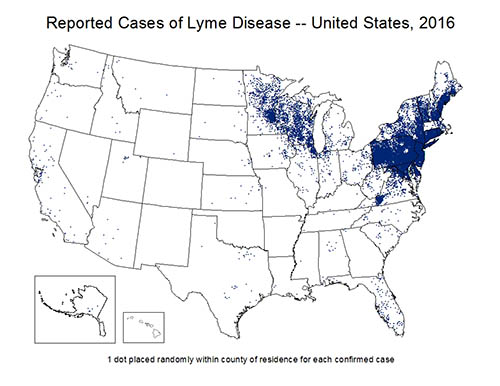
Lyme disease is transmitted by tick bites. The prime environmental reservoirs for the Borrelia species include deer, mice, squirrels, and lizards. In endemic areas, the primary vector for B. burgdorferi is the Ixodes tick. Borrelia species are more prevalent in Europe and the United States, but pathogenic strains have been observed on all continents except for Antarctica. In the United States, Borrelia is commonly found on the East Coast, the Midwest, and Northwestern states; however, most of the clinical cases are concentrated in the Northeast and upper-Midwest United States. Additionally, several studies have demonstrated a low prevalence of potential reservoirs and insect vectors in the Southwest U.S. [5]. Generally, the hot and arid conditions associated with the Southwest are likely inhospitable for known tick vectors [6,7].
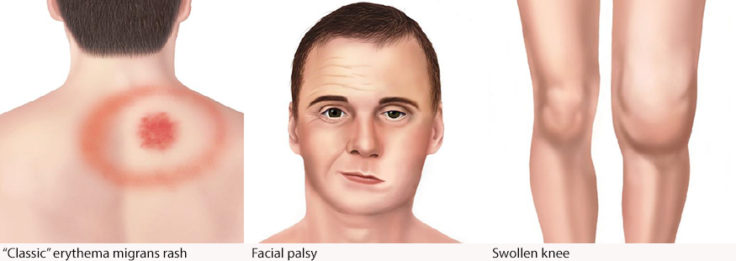
The typical symptoms of Lyme disease may include the classic erythema migrans “bulls-eye” rash, fever, malaise, meningitis, headache, photophobia, joint, and muscle pain.
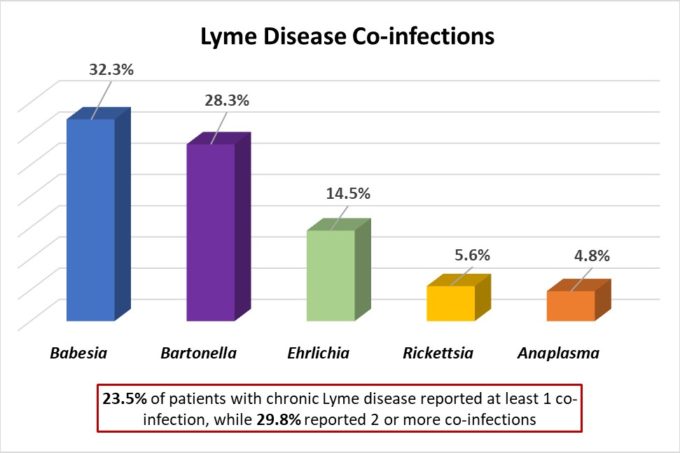
There are several additional infections that can “hitch-hike” with Borrelia species as they share the same insect vector, tick, and mode of transmission, bite [8]. These infections, known as co-infections, include Anaplasma, Ehrlichia, and Babesia species. Frequently these co-infections are overlooked when Lyme disease is suspected; however, these organisms can cause significant illness by themselves as well.
Data adapted from “Severity of chronic Lyme disease compared to other chronic conditions: a quality of life survey“